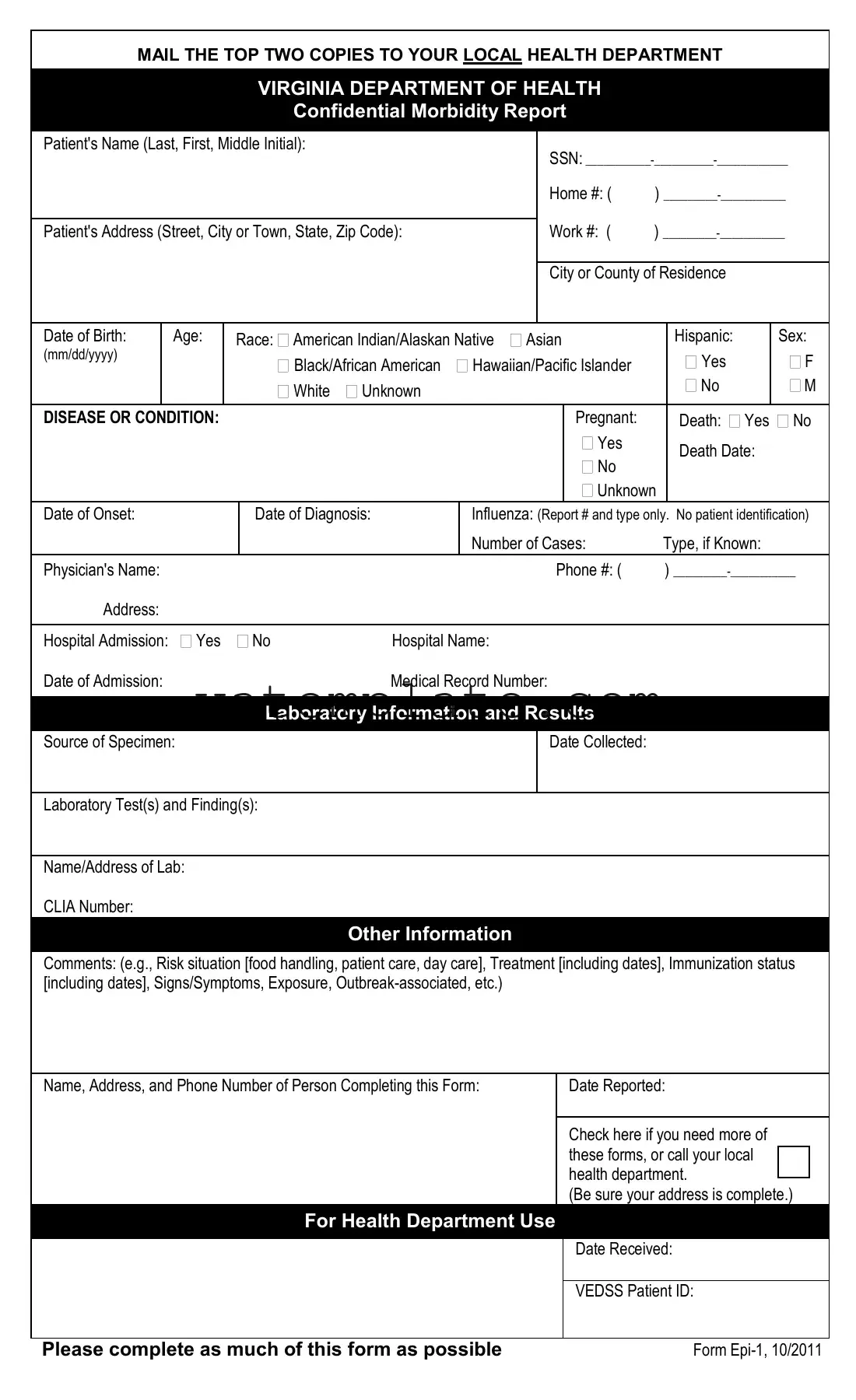
Fill Out a Valid Virginia Epi 1 Template
In the realm of public health, efficient and accurate reporting of diseases and conditions is crucial for timely intervention and control measures. The Virginia Epi 1 form serves as a key instrument in this process, facilitating the structured and confidential communication of morbidity information from healthcare providers to the Virginia Department of Health. This comprehensive document is designed to capture a wide array of data, including the patient's demographic details, the diagnosed disease or condition, relevant laboratory results, and other vital information like exposure or treatment details. It stands as a required submission for a plethora of reportable diseases—ranging from infectious diseases, such as Influenza and Hepatitis, to conditions of public health concern like elevated blood lead levels. The form underscores the mandate by the Code of Virginia and health regulations for disease reporting and control, emphasizing the obligation of healthcare professionals and laboratories to report both suspected and confirmed cases. By meticulously completing and mailing the top two copies of this form to the local health department, healthcare entities partake in a critical task of public health surveillance that underscores the collective effort to monitor, prevent, and control disease outbreaks, thereby safeguarding the community's health and well-being.
Virginia Epi 1 Example
MAIL THE TOP TWO COPIES TO YOUR LOCAL HEALTH DEPARTMENT
VIRGINIA DEPARTMENT OF HEALTH
Confidential Morbidity Report
Patient's Name (Last, First, Middle Initial):
SSN:
Home #: ( )
Patient's Address (Street, City or Town, State, Zip Code):
Work #: ( )
|
|
|
|
|
City or County of Residence |
|
||
|
|
|
|
|
|
|
||
Date of Birth: |
Age: |
Race: American Indian/Alaskan Native |
Asian |
Hispanic: |
Sex: |
|||
(mm/dd/yyyy) |
|
Black/African American |
Hawaiian/Pacific Islander |
Yes |
F |
|||
|
|
|||||||
|
|
White |
Unknown |
|
|
|
No |
M |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
DISEASE OR CONDITION: |
|
|
|
|
Pregnant: |
Death: Yes |
No |
|
|
|
|
|
|
|
Yes |
Death Date: |
|
|
|
|
|
|
|
No |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unknown |
|
|
Date of Onset:
Date of Diagnosis:
Influenza: (Report # and type only. No patient identification)
Number of Cases: |
Type, if Known: |
Physician's Name: |
|
|
Phone #: ( |
) |
Address: |
|
|
|
|
|
|
|
|
|
Hospital Admission: |
Yes |
No |
Hospital Name: |
|
Date of Admission: |
|
|
Medical Record Number: |
|
Laboratory Information and Results
Source of Specimen:
Laboratory Test(s) and Finding(s):
Date Collected:
Name/Address of Lab:
CLIA Number:
Other Information
Comments: (e.g., Risk situation [food handling, patient care, day care], Treatment [including dates], Immunization status [including dates], Signs/Symptoms, Exposure,
Name, Address, and Phone Number of Person Completing this Form:
Date Reported:
Check here if you need more of these forms, or call your local health department.
(Be sure your address is complete.)
For Health Department Use
Date Received:
VEDSS Patient ID:
Please complete as much of this form as possible |
Form |

MAIL THE TOP TWO COPIES TO YOUR LOCAL HEALTH DEPARTMENT
Please report the following diseases (and any other disease or outbreak of public health importance) in the manner required by Sections
Acquired immunodeficiency syndrome (AIDS) Amebiasis *
ANTHRAX * 
Arboviral infection (e.g., dengue, EEE, LAC, SLE, WNV) *
BOTULISM * BRUCELLOSIS *  Campylobacteriosis * Chancroid * Chickenpox (Varicella) * Chlamydia trachomatis infection *
Campylobacteriosis * Chancroid * Chickenpox (Varicella) * Chlamydia trachomatis infection *
CHOLERA * 
Cyclosporiasis *
DIPHTHERIA * 
DISEASE CAUSED BY AN AGENT THAT MAY HAVE BEEN USED AS A WEAPON
Ehrlichiosis/Anaplasmosis *
Escherichia coli infection, Shiga  ^ Giardiasis *
^ Giardiasis *
Gonorrhea * Granuloma inguinale
HAEMOPHILUS INFLUENZAE INFECTION, INVASIVE *  Hantavirus pulmonary syndrome *
Hantavirus pulmonary syndrome *
Hemolytic uremic syndrome (HUS)
HEPATITIS A *
Hepatitis B (acute and chronic) * Hepatitis C (acute and chronic) * Hepatitis, other acute viral
Human immunodeficiency virus (HIV) infection * Influenza * #
(report INFLUENZA A, NOVEL VIRUS immediately) 
Lead, elevated blood levels * Legionellosis *
Leprosy (Hansen disease) Listeriosis * 
Lyme disease * Lymphogranuloma venereum Malaria *
MEASLES (RUBEOLA) * MENINGOCOCCAL DISEASE * 
MONKEYPOX * Mumps *
MYCOBACTERIAL DISEASES (INCLUDING AFB),
 (IDENTIFICATION OF ORGANISM) AND DRUG SUSCEPTIBILITY
(IDENTIFICATION OF ORGANISM) AND DRUG SUSCEPTIBILITY
Ophthalmia neonatorum
OUTBREAKS, ALL (including, but not limited to, foodborne,
PERTUSSIS *  PLAGUE *
PLAGUE * 
POLIOVIRUS INFECTION, INCLUDING POLIOMYELITIS *  PSITTACOSIS *
PSITTACOSIS *
Q FEVER * 
RABIES, HUMAN AND ANIMAL * Rabies treatment,
RUBELLA, INCLUDING CONGENITAL RUBELLA SYNDROME * Salmonellosis * 
SEVERE ACUTE RESPIRATORY SYNDROME (SARS) * Shigellosis * 
SMALLPOX (VARIOLA) * Spotted fever rickettsiosis * Staphylococcus aureus infection
invasive 
Streptococcal disease, Group A, invasive or toxic shock *  Streptococcus pneumoniae infection, invasive, in children <5 years
Streptococcus pneumoniae infection, invasive, in children <5 years
of age *
Syphilis (report PRIMARY and SECONDARY immediately) * Tetanus
Toxic
TUBERCULOSIS (TB), ACTIVE DISEASE *  Tuberculosis infection in children <4 years of age
Tuberculosis infection in children <4 years of age
TULAREMIA * TYPHOID/PARATYPHOID FEVER *  UNUSUAL OCCURRENCE OF DISEASE OF
UNUSUAL OCCURRENCE OF DISEASE OF
PUBLIC HEALTH CONCERN VACCINIA, DISEASE OR ADVERSE EVENT * VIBRIO INFECTION *
VIRAL HEMORRHAGIC FEVER * YELLOW FEVER *
Yersiniosis * 
Report all conditions to your local health department when suspected or confirmed. Those in UPPER CASE must be reported immediately by the most rapid means available. All others must be reported within 3 days.
*These conditions are reportable by directors of laboratories. In addition, these and all other conditions except mycobacterial disease (other than TB) and invasive MRSA infection are reportable by physicians and directors of medical care facilities. Reports may be by computer- generated printout,
 A laboratory identifying evidence of these conditions shall notify the health department of the positive culture and submit the initial isolate to the Virginia Division of Consolidated Laboratory Services (DCLS) or, for TB, to DCLS or other laboratory designated by the Board.
A laboratory identifying evidence of these conditions shall notify the health department of the positive culture and submit the initial isolate to the Virginia Division of Consolidated Laboratory Services (DCLS) or, for TB, to DCLS or other laboratory designated by the Board.
^Laboratories that use a Shiga toxin EIA methodology but do not perform simultaneous culture for Shiga
#Physicians and directors of medical care facilities should report influenza by number of cases only (report total number per week and by type of influenza, if known); however, individual cases of influenza A novel virus must be reported immediately by the most rapid means available.
Note: 1. Some
2.Cancers are also reportable. Contact the VDH Virginia Cancer Registry at (804)
Virginia Department of Health
Office of Epidemiology
P.O. Box 2448, Suite
Form Properties
| Fact | Detail |
|---|---|
| Form Name | Virginia Epi-1 Form |
| Purpose | Confidential Morbidity Report |
| Recipient | Local Health Department |
| Governing Law | Sections 32.1-36 and 32.1-37 of the Code of Virginia and 12 VAC 5-90-80 and 12 VAC 5-90-90 of the Board of Health Regulations for Disease Reporting and Control |
| Information Collected | Patient's Name, SSN, Contact Information, Disease or Condition, Physician's Information, Hospital Admission, Laboratory Information and Results, Other Information related to the patient's condition |
| Reportable Diseases | List includes but not limited to AIDS, Anthrax, Influenza, Measles, Rabies, etc. |
| Reporting Urgency | Conditions in uppercase must be reported immediately; all others within 3 days. |
| Special Instructions | Enter as much information as possible on the reporting form. |
| Reporting Method | May be reported by computer-generated printout, Epi-1 form, CDC surveillance form, or secure electronic transmission as agreed with VDH. |
| Additional Notes | Some healthcare-associated infections and cancers are also reportable. |
Steps to Filling Out Virginia Epi 1
Upon encountering a public health situation that warrants notification, healthcare providers and laboratories must accurately complete and promptly submit the Virginia Epidemiology Form 1 (Epi 1), adhering to the guidelines set forth by the Virginia Department of Health. This form plays a pivotal role in the monitoring and managing of health events in the state, ensuring timely interventions are made to safeguard public health. Here are the steps to accurately fill out the Virginia Epi 1 form:
- Begin with the Patient's Name section by entering the last name, first name, and middle initial of the patient involved in the reported condition.
- Fill in the Social Security Number (SSN) of the patient across the provided dashes.
- Enter the patient's home telephone number, including the area code, in the space provided for Home #.
- Under Patient's Address, provide the street, city or town, state, and zip code where the patient resides.
- When available, furnish the patient's work telephone number, including area code, in the Work # field.
- Specify the City or County of Residence to help localize the report within Virginia.
- Record the patient's Date of Birth, Age, Race, and Sex with the appropriate selections or information in the respective sections.
- Indicate whether the patient is Pregnant or if the notification is reporting a Death, and include the Death Date if applicable.
- Detail the Disease or Condition being reported. Input the Date of Onset and Date of Diagnosis to provide timelines of the illness.
- If reporting Influenza, specify the Number of Cases and the Type, if Known.
- In the section provided, input the Physician's Name, Phone #, and Address who is reporting or managing the patient's condition.
- Mark the appropriate response for Hospital Admission and provide the Hospital Name, and Date of Admission if admitted, along with the Medical Record Number.
- Under Laboratory Information and Results, describe the Source of Specimen, list all Laboratory Test(s) and Finding(s), and the Date Collected. Include the Name/Address of Lab and its CLIA Number.
- In the Other Information Comments section, add any relevant details like risk situations, treatment provided with dates, immunization status with dates, signs/symptoms, exposures, or if the report is related to an outbreak.
- Finally, at the bottom of the form, the Name, Address, and Phone Number of Person Completing this Form must be filled in, along with the Date Reported. Check the box if you require more forms.
After completing the form with as much precision and detail as possible, mail the top two copies to your local health department. This ensures the information is processed in a timely manner, facilitating appropriate public health responses and interventions.
FAQ
What is the purpose of the Virginia Epi 1 form?
The Virginia Epi 1 form is used by healthcare providers to report certain diseases, conditions, and outbreaks that are of public health importance to the Virginia Department of Health. Its main purpose is to ensure that the health department is informed about illnesses that might require public health intervention or monitoring. Reporting these conditions helps in tracking, preventing, and controlling the spread of diseases within the state.
Who needs to fill out the Virginia Epi 1 form?
This form must be completed by physicians, directors of medical care facilities, and laboratory directors. While all mentioned diseases and conditions must be reported, certain ones identified in all caps on the form require immediate notification by the fastest means possible. Others should be reported within three days of identification.
What illnesses are reportable on the Virginia Epi 1 form?
Many disease conditions are reportable, ranging from infectious diseases like influenza, Hepatitis A, B, and C, and Measles (Rubeola), to more severe conditions such as Anthrax, Ebola, and Rabies. The form lists both diseases that must be reported immediately and those that can be reported within a three-day window.
How are the diseases reported?
Reports can be made via computer-generated printouts, the Epi-1 form itself, CDC surveillance forms, or, upon agreement with the Virginia Department of Health (VDH), by secure electronic transmission. Immediate reportable diseases should be communicated through the fastest means available, including phone calls directly to the local health department.
What is the role of laboratories in reporting diseases?
Laboratories play a crucial role in disease reporting. They are required to notify the health department about positive cultures for the listed conditions and submit the initial isolate to the Virginia Division of Consolidated Laboratory Services (DCLS). For certain tests, like those identifying Shiga toxin-producing E. coli without concurrent culture tests, the laboratory must forward the positive specimen to DCLS for further confirmation and characterization.
Is there any confidentiality concern with reporting diseases?
Yes, the Virginia Epi 1 form is a confidential morbidity report. The information collected is used exclusively for public health purposes, such as tracking, controlling, and preventing disease. Patient privacy is protected under health information privacy laws, and the data is handled with strict confidentiality protocols to safeguard individual privacy.
What information is required on the Epi 1 form?
The form requires detailed patient information, including name, social security number, contact information, and demographic details like age, race, and sex. It also asks for the disease or condition being reported, relevant dates such as onset and diagnosis, and any laboratory test results. Healthcare providers are encouraged to provide as much information as possible to aid in public health efforts.
Where do I submit the completed Virginia Epi 1 form?
The top two copies of the completed form must be mailed to the local health department of the patient's city or county of residence. The address for the local health department can typically be found on the Virginia Department of Health's website or by contacting the VDH directly.
Can I request additional forms if needed?
Yes, if more forms are needed, you can check the request box on the Epi 1 form itself or call your local health department. They will provide you with additional forms so that you can continue to report any diseases or conditions as mandated.
Common mistakes
When filling out the Virginia Confidential Morbidity Report Form (Epi 1), it's crucial to avoid common mistakes to ensure accurate and timely disease reporting. Given the importance of this process for public health monitoring and response, let's explore eight common errors to watch out for:
- Omitting Patient's Social Security Number (SSN): The SSN is a crucial identifier that helps the health department accurately track and report diseases. Ensure the full SSN is included, using the format xxx-xx-xxxx.
- Incomplete Patient Information: Every detail in the patient's information section is vital. Missing out on the full name, including the middle initial, or the complete address including zip code, can lead to reporting errors or delays.
- Incorrect or Missing Contact Information: Both home and work phone numbers should be provided when available, including the area code. This information is essential for any necessary follow-up.
- Misclassification of Disease or Condition: Ensure the disease or condition reported is accurately classified according to the guidelines provided by the Virginia Department of Health. This includes checking the right boxes and, if reporting an outbreak, specifying the disease if known.
- Laboratory Information Errors: The laboratory section must include the source of the specimen, the name and address of the lab, and the laboratory test(s) and finding(s). Any errors or omissions in this section can lead to misinterpretation of the patient's condition.
- Incomplete Reporting of Disease Onset and Diagnosis Dates: Providing accurate dates for both the onset of symptoms and diagnosis are critical for disease tracking and management of public health responses.
- Failing to Report Pregnancy Status: For diseases that can affect pregnancy outcomes or be transmitted from mother to child, it is essential to accurately report the pregnancy status.
- Not Utilizing the Comments Section Effectively: The comments section is there for additional important information, such as exposure details, signs and symptoms, and any treatment given. Leaving this section blank or not fully utilizing it can result in incomplete reporting.
Remember, accurate and complete reporting on the Epi 1 form is essential for effective public health surveillance and response. By avoiding these common mistakes, individuals can contribute to the health and safety of the community.
Documents used along the form
When managing a Virginia Epi 1 form for reportable diseases or conditions, healthcare and lab professionals often need to include additional documentation to provide a full picture of the situation to the Virginia Department of Health. These documents not only offer a detailed look into the patient's condition but also help in the broader surveillance efforts aimed at controlling and preventing diseases. Understanding these supplementary forms and documents can streamline the reporting process and ensure compliance with state health regulations.
- Laboratory Test Results: Detailed reports from laboratory tests that identify the disease or condition. These results can confirm the diagnosis and are crucial for the Epi 1 form's accuracy.
- Patient Consent Forms: Documents that show the patient has agreed to have their information shared for public health purposes. These forms are important to protect patient privacy rights.
- Physician's Notes: Notes or medical observations made by the attending physician. They provide context to the diagnosis and can include symptoms, disease progression, and response to treatment.
- Epidemiological Questionnaires: These questionnaires collect detailed information about potential exposure sources, travel history, and contacts, which is critical in investigating and controlling outbreaks.
- Immunization Records: Records that detail any vaccinations the patient may have received. This information is vital in cases of vaccine-preventable diseases.
- Treatment Records: Documents detailing the course of any treatments administered to the patient. This can include medications given, hospitalization records, and follow-up plans.
- Contact Tracing Forms: These forms help in identifying and monitoring individuals who have been in close contact with the patient. They are essential tools in preventing disease spread.
- Death Certificate: If the report involves a deceased patient, a copy of the death certificate may be required to provide official cause of death and other relevant data.
- Reportable Disease Confidential Case Report Form: Some conditions require this specific form in addition to the Virginia Epi 1, offering more detailed information on particular diseases.
In summary, the Virginia Epi 1 form serves as a critical component in public health surveillance, but it is often not alone. By accompanying it with relevant forms and documents, health professionals ensure a thorough and accurate report to the state's Department of Health. This comprehensive approach enables effective monitoring, control, and prevention of diseases, safeguarding the health of the community.
Similar forms
The Virginia Epi 1 form is similar to other public health forms used across the United States, designed for reporting various diseases and conditions. These forms often share common features, such as sections for personal information, details about the disease or condition being reported, laboratory results, and information on the healthcare provider. While each form has its unique aspects per state regulations and specific diseases, the overarching goal remains the same—to facilitate the efficient reporting and tracking of diseases to monitor and control public health threats.
The CDC 50.42A form, used for the National Notifiable Diseases Surveillance System (NNDSS), resembles the Virginia Epi 1 form in several ways. Both documents require detailed information about the patient, including demographics such as age, sex, and race, as well as clinical data like the date of onset, diagnosis, and laboratory test results. Additionally, they each have sections dedicated to the healthcare provider’s and reporting entity's contact details. The main difference lies in the CDC 50.42A form’s broader use, serving as a standard reporting form for notifiable diseases at a national level, whereas the Virginia Epi 1 form is tailored for reporting within the state of Virginia.
The California Confidential Morbidity Report (CMR) form is another example. Similar to the Virginia Epi 1 form, the CMR collects detailed patient information, disease or condition specifics, and laboratory findings. Both forms are critical for local health departments to ensure prompt public health responses, including outbreak management and disease prevention efforts. The primary distinction arises from the specific diseases listed for reporting, which may vary between the forms due to different public health priorities and disease prevalence in California compared to Virginia.
In essence, while the Virginia Epi 1 form shares many characteristics with other state and national public health reporting documents, subtle differences exist to accommodate the unique epidemiological needs of each jurisdiction. These forms are vital components of a comprehensive public health surveillance system, ensuring that health departments have the necessary information to protect public health effectively.
Dos and Don'ts
When filling out the Virginia Epi 1 form, it is important to adhere to certain guidelines to ensure accurate and complete reporting. Here is a list of things you should and shouldn't do:
- Do:
- Ensure all patient information is filled in, including the patient's full name, Social Security Number, contact information, and address. Incomplete information can lead to delays in processing.
- Include detailed information about the disease or condition being reported, such as the date of onset, diagnosis, and any relevant laboratory results.
- Report the disease or condition as specifically required by the sections of the Code of Virginia and Board of Health Regulations.
- Provide information about the patient's demographic details such as age, race, and sex, since these can be crucial for epidemiological studies.
- Check the correct boxes for questions such as "Pregnant," "Death," "Hospital Admission," to clearly communicate the patient's status.
- Don't:
- Leave sections blank unless they truly do not apply to the case you are reporting. If unsure, it's better to provide as much information as possible or explain why the information is not available.
- Forget to include your own name, address, and phone number as the person completing the form. This is critical in case follow-up information is needed.
- Use abbreviations or medical jargon not widely understood. Keep in mind that the form should be clear to anyone reading it, not just medical professionals.
- Delay sending the top two copies to your local health department. Timely reporting can be essential for public health responses.
- Fail to request more forms if you are running low. It's important to have a sufficient supply to continue reporting without interruption.
By following these guidelines, individuals can ensure that the information provided on the Virginia Epi 1 form is complete, accurate, and submitted in a timely manner, helping health authorities to effectively monitor and manage public health concerns.
Misconceptions
There are several misconceptions about the Virginia EPI-1 form that need clarification to ensure accurate and efficient reporting of diseases. Understanding these misconceptions is crucial for healthcare providers, laboratory personnel, and public health officials. Below is a list of common misconceptions and their explanations.
Only contagious diseases need to be reported: The Virginia EPI-1 form is used to report a wide range of conditions, not just contagious diseases. It includes non-communicable diseases and conditions of public health concern as well.
The form is only for doctors to complete: While physicians are primarily responsible for reporting, laboratory directors, and certain other healthcare professionals can also complete and submit the form for reportable conditions.
Reporting is voluntary: Reporting diseases using the Virginia EPI-1 form is mandated by law for certain conditions outlined in the document. It's not a voluntary activity.
All sections must be fully completed for the report to be accepted: While it’s important to provide as much detail as possible, reports should still be submitted even if some sections of the form are incomplete. Vital information should always be included.
Electronic reporting is not an option: Reports can be submitted electronically, in agreement with the Virginia Department of Health (VDH), in addition to using the EPI-1 form or CDC surveillance forms.
Only current residents of Virginia need to be reported: Any individual diagnosed or treated in Virginia for a reportable condition should be reported, regardless of their residency status.
Reporting is only necessary for confirmed cases: Suspected cases of certain diseases, particularly those of high urgency or seriousness, should be reported even before confirmation is available.
Influenza must be reported for individual cases: Influenza reporting is generally done by total number of cases per week and type, if known, except for novel virus strains, which must be reported immediately and individually.
The EPI-1 form is outdated and no longer in use: The EPI-1 form is updated regularly to reflect current reporting needs and remains a critical tool for disease reporting in Virginia.
All reportable conditions must be reported within the same timeframe: The urgency of reporting varies by condition. Some require immediate report by the most rapid means available, while others must be reported within 3 days.
Understanding these misconceptions is essential for timely and effective disease reporting, which is crucial for monitoring public health and implementing necessary interventions.
Key takeaways
Filling out and utilizing the Virginia Epi 1 form is a critical process conducted by healthcare providers and laboratories to report certain diseases and conditions to the Virginia Department of Health (VDH). Understanding the key details regarding this form can enhance the accuracy and efficiency of disease reporting, which is vital for public health monitoring, intervention, and management. Here are five key takeaways about the Virginia Epi 1 form:
- The Epi 1 form serves as a confidential morbidity report, designed for the reporting of specific diseases and conditions as mandated by Sections 32.1-36 and 32.1-37 of the Code of Virginia, and regulations 12 VAC 5-90-80 and 12 VAC 5-90-90 of the Board of Health Regulations for Disease Reporting and Control.
- It is essential for users to report both suspected and confirmed cases. The diseases and conditions that need to be reported include a wide spectrum, from influenza to more severe conditions like hepatitis, measles, and rabies. Those spelled out in uppercase on the form are to be reported immediately by the fastest means available, while others should be reported within three days.
- Laboratories play a significant role in this reporting process. For identified conditions, laboratories are required not only to notify the health department of the positive culture but also, in certain cases, to forward specimens to the Virginia Division of Consolidated Laboratory Services (DCLS) for further analysis or confirmation.
- The form requests detailed patient information, including demographics, disease or condition specifics, laboratory results, and information regarding treatment or hospitalization. It also includes sections for comments which can cover risk situations, symptoms, and exposure details, enhancing the depth of information provided to the health department.
- Completing the form with as much information as possible and mailing the top two copies to the local health department as instructed ensures timely and accurate reporting. This is paramount for the health department's efforts in tracking, preventing, and controlling the spread of diseases throughout the state.
By adhering closely to the guidelines and requirements specified for the Virginia Epi 1 form, healthcare providers and laboratories can help maintain high standards of public health and safety in the community.
Other PDF Forms
Virginia State Application - Encourages the indication of available start dates, providing agencies with insights into the applicant's transition availability.
Virginia State Police Crash Reports - Recording of the vehicle's condition post-crash, including damage and functionality, assists in legal and insurance assessments.
Virginia Real Estate Contract for Sale by Owner - It highlights essential clauses like the deposit amount, financing contingency, and the settlement procedure to facilitate a smoother transaction between buyer and seller.